BIOENGR 167L - Bioengineering Laboratory
BE 167L - Bioengineering Laboratory
Lab 4: Microcontact Printing and Contact Angle Measurements
Prelab reading
Watch the lab primer video.
Micro-contact printing
You will pattern a polystyrene well with different concentrations of your fluorescein-BSA from Lab 3. Patterned surfaces are used often in cell biology to probe cell behavior on substrates and cell response to adsorbed chemical signals. This is a useful tool for creating patterns of adhesive proteins that allow specific cell types to adhere in specific patterns. Creating patterned cultures of single cell types or patterned co-cultures of multiple cell types can help researchers study behavior of cells under controlled conditions. Limiting variables by controlling culture conditions allows for more rigorous testing of hypotheses.
Preparation
Reagents
- Fluorescein-BSA from Lab 3
- Phosphate buffered saline (PBS) pH 7.4
- Deionized (DI) water
- Your PDMS stamps in the molds from lab 2
Supplies
- 1.5–2 mL microcentrifuge tubes
- 20 mL scintillation vials
- A large or medium petri dishes for storing stamps is desired
- 1 6-well non-sterile, non-tissue culture plate
- Razor blade or scalpel, thin spatula
- Aluminum foil
- Plastic transfer pipettes
- Pipettes and tips
Equipment
- Plasma Cleaner
- Fluorescent Microscope
- Camera (phone or otherwise)
Safety
In addition to your usual protective equipment, (lab coat, gloves safety glasses), you should be aware of three things. Remember that your fluorescein-BSA solution contains DMSO, an organic solvent. Also, do not look directly into the plasma cleaner for prolonged periods of time. When using the fluorescent microscope, do not look directly at the beam of light from the fluorescent lamp for prolonged periods of time.
Procedure
Prepare solutions for micro-contact printing
- Retrieve your vial of fluorescein-BSA from the fridge.
- Retrieve your cast molds and use a thin spatula or scalpel blade to free all the edges from the petri dish walls. Do not throw away the white acrylic cast molds, give them to your TA to save.
- Choose one of your molds (#1) to use for protein stamping with plasma treatment and the second mold (#2) for contact angle measurements. If one of the molds is damaged during the retrieval process, use that as mold #2 for contact angle measurements.
- Some groups can start the micro-contact printing first by proceeding to Plasma surface treatment with mold #1 while other groups can start with Contact angle measurements
Plasma surface treatment
Note: Make sure that your TA is present when you are operating the plasma cleaner equipment.
- Place your stamp #1 on the tray located somewhere around the plasma cleaner with the features up (flat side down). Place the tray inside the plasma cleaner. Multiple groups may use the plasma cleaner at once to save time. Your TA may coordinate usage of the plasma cleaner for efficiency.
- The Swagelok valve has three directions (closed, open to atmosphere, and adjustably open). Turn the valve so that it points toward neither open setting (down).
- Press the pump button and gently push then pull the door to make sure it is closed. Wait 2 minutes and read the next steps.
- After two minutes press the power button. After several seconds a pinkish light should appear in the window. 5. Turn the valve so that the arrow points toward the adjustably open setting. The knob should be set to the optimal setting. If the pink light does not appear brighter then turn the knob a few degrees in either direction until the light is at its peak intensity.
- At the peak intensity, wait 2 minutes.
- Turn off the power and the pump.
- Turn the valve so that it is closed then slowly open the valve to atmosphere. It is important that you do this slowly so that incoming air does not flip your stamp over.
- Remove your stamps from the cleaner.
Micro-contact printing (µCP)
- Fill a 20 mL scintillation vials with tap water. This weight will be used to ensure even contact of your stamp with the substrate.
- Soon after removing your stamp #1 from the plasma cleaner, pipet 100 µL of your Fluorescein-BSA solution onto each “window pane” of your stamp (so 400 µL total). Using the tip of the pipette, spread the protein solutions, so that all the patterns are covered with protein. If you need more solution to cover all of the patterns, pipet more solution onto the surface. It is ok if some solution falls into the trough regions of the surface.
- Shield the stamps from light and allow protein to adsorb to the stamp for 5 minutes.
- Take a kimwipe and without touching the top of the stamps remove the fluid (touch the liquid, but not the stamp). Be sure to thoroughly wick fluid from between the features as well.
- Let the stamps air dry for a couple minutes you want the surface to be moist but not wet. This is because you do not want excess fluid while stamping, as this will negatively influence your protein stamping.
- You will be provided with a 6-well plate. Place the stamp with the features down at the middle of a well. Once the stamp is down you will not want to move it. Place the scintillation vial that you prepared on top of the stamp. You will not want to adjust this either. Let the protein transfer from the stamp to the dish for 3 minutes.
- In a swift, even motion, remove your weight and stamp from the surface. Avoid peeling the stamp as this will result in uneven patterns. By all means necessary do not slide the stamp as this will result in protein shearing and you may not see any features. Remember which well had been stamped.
- Look at your dish under the fluorescent microscope to document the pattern transfer and relative intensities of the patterns according to treatment condition and concentration (refer to previous lab protocols for fluorescent microscopy instruction if you need help). Take images that demonstrate a contrast between the protein patterned and un-patterned regions and that illustrate the accuracy of the pattern. Take a minimum of three images at different objective magnifications.
Contact angle measurements
- Cut mold #2 in half so that there are two whole “window panes” on each half.
- Plasma treat one of the pieces according to the procedure outlined above.
- Using a pipette, place a 10 µL drop of DI water onto each piece of the stamp. Take care to use the plasma treated side of the appropriate piece. You are comparing water contact angle between a plasma treated surface and non-treated surface of PDMS.
- Place these stamps on an elevated surface, such as a tube rack or textbook, with a dark solid background behind them.
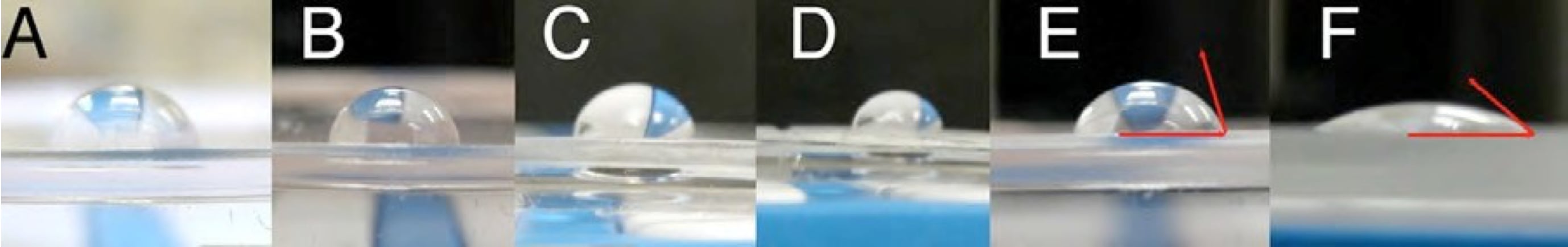
- Using your cell phone camera (or a camera provided by your TA if you do not have one), get close to your samples directly from the side and take a picture with the surface of the stamp as the horizon so that you can measure the angle of the water bead from the surface as accurately as possible. You may take separate pictures of each surface. Refer to the figure below for tips on how to take a good picture for contact angle measurements.
- Repeat steps 3-5 three times with your two surfaces to generate raw data points that you can average. Do this on different spots of the same surface rather than just taking multiple pictures of the same drop. Always use the same size drop.
Note: The surface treatment lasts for only half an hour. Make sure you take pictures of your PDMS stamps right after treatment.