BIOENGR 167L - Bioengineering Laboratory
BE 167L - Bioengineering Laboratory
Lab 6: Gauging Confluency and Growth Kinetics
Prelab reading
In addition to the text below, please read the ATCC Animal Cell Culture Guide posted on Bruin Learn.
Being able to look at a flask of cells and estimate a rough order of magnitude for the number of cells is a useful skill. You may be growing cells for an experiment and need to estimate how long it will take to grow enough cells. Also estimating the confluency of a flask and how much time you might have before they become confluent is important for knowing when to passage cells before they become too confluent. Once they become confluent they may stop dividing and enter a different phase of the cell cycle, which may not be desirable for your experiment. You would typically passage cells when they reach 80-90% confluency. 100% confluency means that cells cover 100% of the available surface area. Look at the figures below for an idea of what cells look like at different densities on a culture surface.
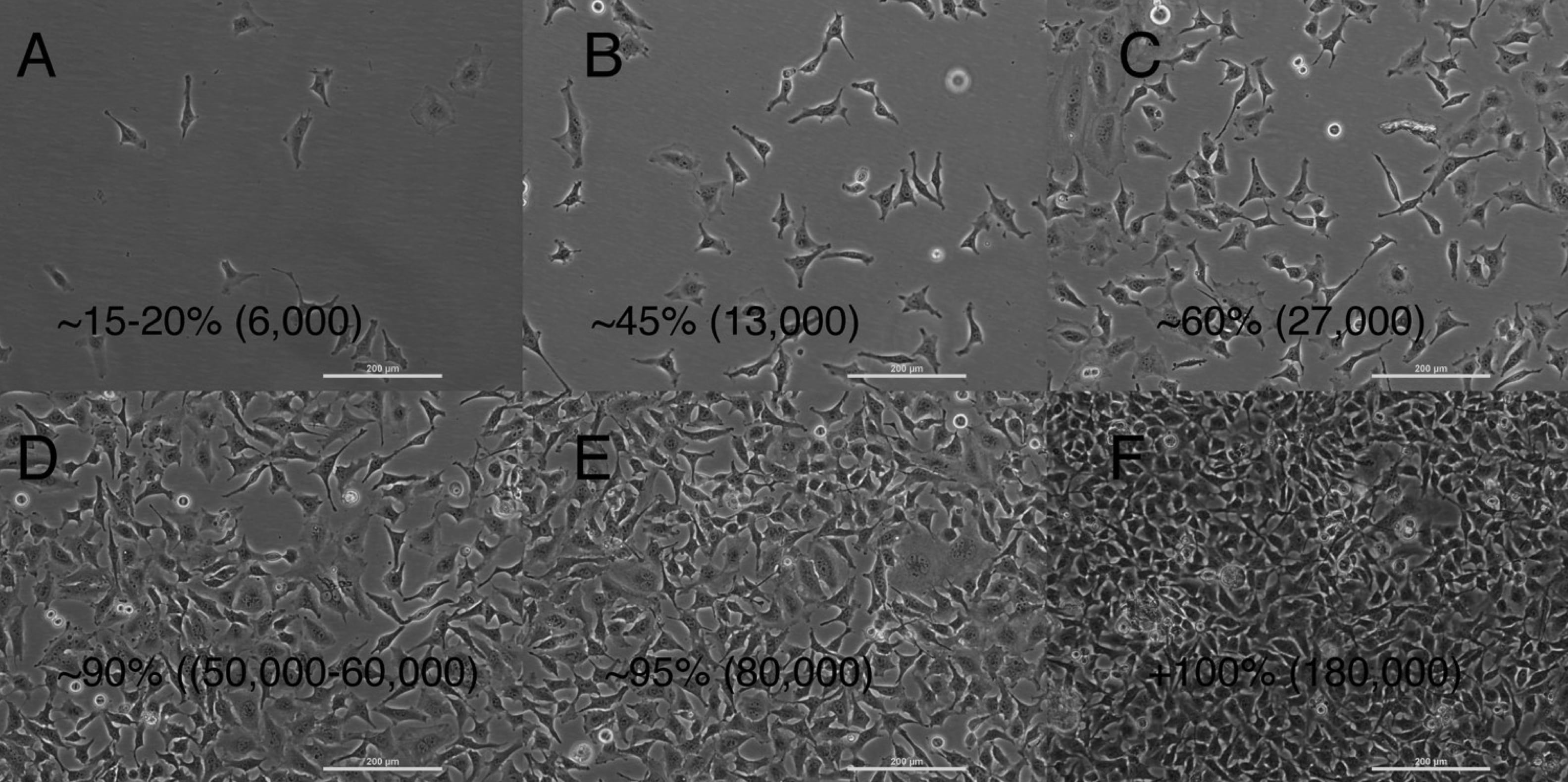
Different densities of cells are shown in this series of images from a phase contrast microscope in the teaching lab. These cells were cultured in tissue culture flasks you will be using and were counted before and after plating using a hemocytometer. The estimated confluencies are given in percentages and the rough number of cells per cm2 are given in parentheses.
Watch the lab primer video.
Gauging confluency
You will observe the different densities of your cells under the microscope to help you correlate what you see with a relative number of cells present. Take pictures that are representative of the overall density that you observe.
Preparation
Reagents
- None
Supplies
- Your previously seeded cell culture flasks
Equipment
- Phase contrast microscope
Safety
Wear your safety glasses, gloves, and lab coat.
Procedure
- Remove your 6 well plate of cells from the incubator
- Look at each of them under the EVOS microscope using phase contrast, noting the uniformity or non-uniformity of distribution of the cells.
- Take a representative photo from each of your wells.
- Wipe the flasks with 70% ethanol prior to putting it back in the incubator, unless you are ready to begin the next section.
Growth kinetics
You will harvest your cells from the 6 well plate and the T25 flask and count them with a hemocytometer so that you can compare what the flasks looked like to how many cells were actually in the flasks. Once you have a feel for the number of cells you are looking at, you can make better judgments during your future experiments.
Preparation
Reagents
- Complete medium from previous lab
- Sterile DPBS from previous lab
- Trypsin
- Your 6 well plate and 1 T25 flask of cells from the previous lab
Supplies
- Serological pipettes of 5 mL to 25 mL in the BSC
- Pipettes and tips for 20–200 µL
- 4 15 mL centrifuge tubes
- 4 microcentrifuge tubes
- 1 new T25 flask
- 1 6-well cell culture plate
Equipment
- Biological safety cabinet (BSC)
- Pipet-aid
- Phase contrast microscope
- Centrifuge
- CO2 cell culture incubator
- Hemocytometer and cell counter
Safety
Wear your safety glasses, gloves, and lab coat. Follow the sterile cell culture techniques you learned in the last lab and be careful of your biohazardous waste disposal procedures.
Procedure
- Follow the same procedure as the last lab to trypsinize each of your wells and your T25 into separately labeled 15 mL centrifuge tubes. Share the flasks among your team members so that each student gets practice.
- A brief re-cap of the procedure from the last lab:
- Aspirate the medium from the flask and rinse twice with DPBS
- Add trypsin, cap, and place in the incubator for 3-5 minutes. Check the flask under the microscope to see when cells are lifted. Agitate if needed.
- Add complete medium to the trypsin to deactivate the enzyme.
- Pipette the cell suspension gently up and down to detach cells from each other and the flask. Use the serological pipette to measure your total volume.
- Transfer to a 15 mL centrifuge tube and collect a small known volume for counting on the hemocytometer.
- Before centrifuging your 15 mL tubes, remove 200 µL from each tube to a separately labeled microcentrifuge tube, keeping track of what densities are in each tube.
- While you are spinning down your cells, count the cells in your microcentrifuge tubes to determine the total number of cells in each of your 15 mL tubes.
- Prepare a 6-well cell culture plate with 3 mL of fresh complete medium in each well for your lab next week.
- You may pool your cells from your 15 mL centrifuge tubes or just use one tube as your source. Resuspend your cells in the appropriate amount of medium to make a 1 million cells/mL suspension.
- In your 12-well plate, seed the top row with 1500 cells/cm2, the bottom row with 5000 cells/cm2, then seed the bottom row with 10000 cells/cm2.
- Re-plate 1 T25 flask with at least 150,000 cells total.
- You may discard your left-over cells by aspirating them.